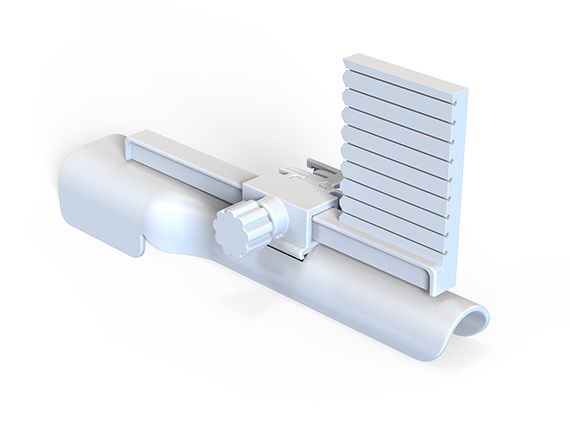
GTK153
The transperineal biopsy needle guide is a disposable device that ensures precise needle placement during prostate biopsies under ultrasound guidance. Its grid system aligns with imaging coordinates, improving accuracy, reducing complications, and enhancing diagnostic yield. Sterile and single-use, it prevents cross-contamination, making it essential for safe, effective procedures.
US-Guided Transperineal Prostate Procedures and Biopsies
- Sterile & Disposable Design: Ensures patient safety by minimizing the risk of infection and cross-contamination.
MR Fusion Compatibility: It features a sensor head housing that seamlessly integrates with MR fusion-guided biopsy systems, enhancing precision in targeted procedures.
Needle Compatibility: It is compatible with 15G-17G coaxial needles and 16G-18 G biopsy needles, offering flexibility for various clinical requirements.
- Regulatory Compliance: CE-certified, MHRA registered and 510(k) FDA-cleared, meeting stringent medical device standards for safety and performance.